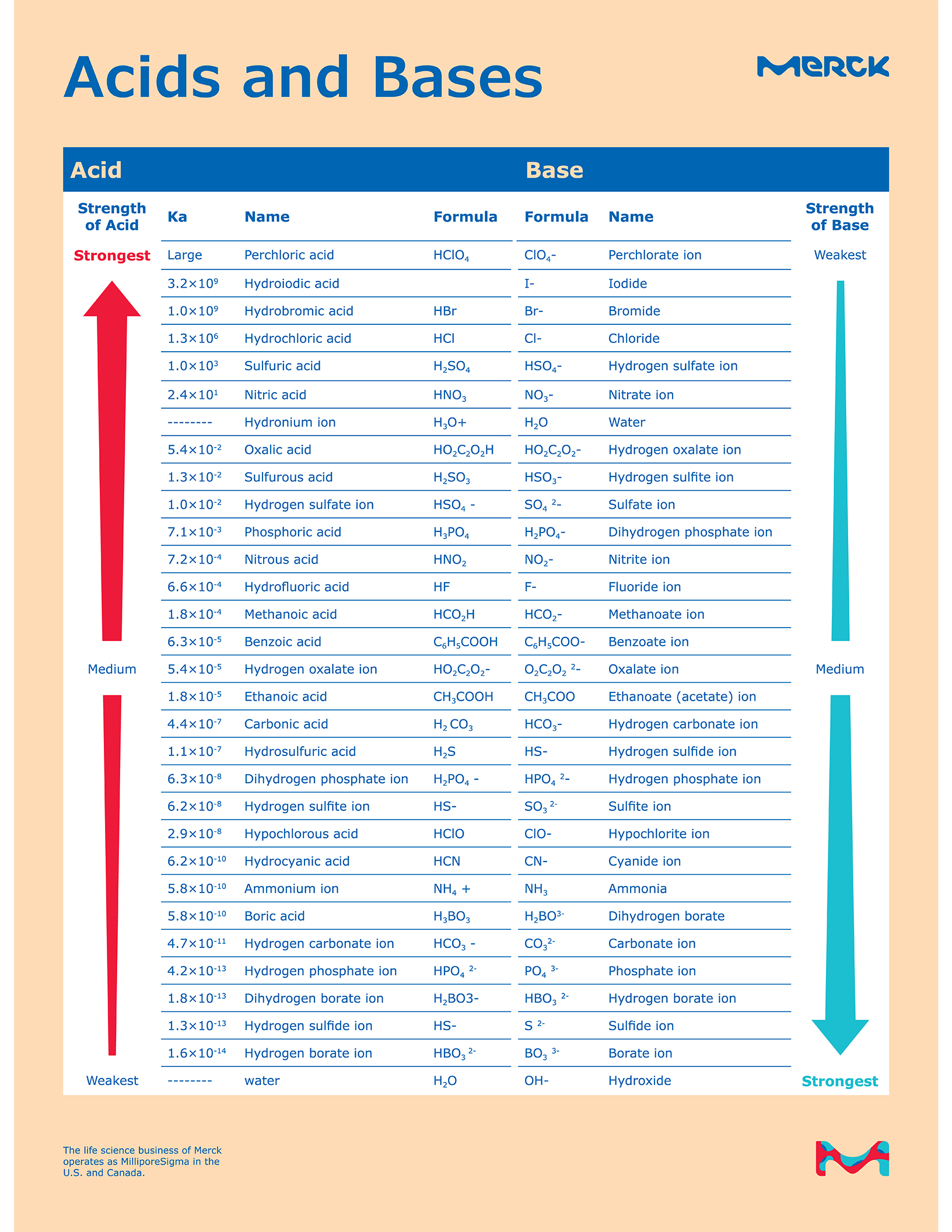
Acid Base Chart
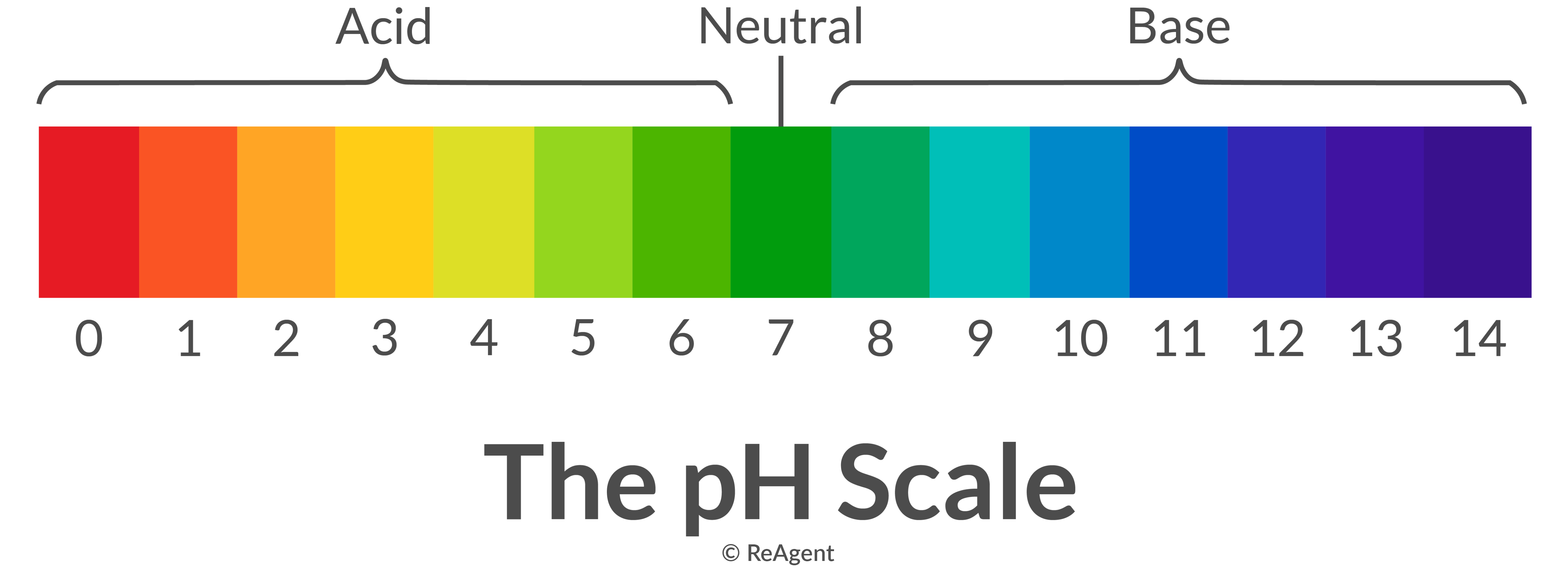
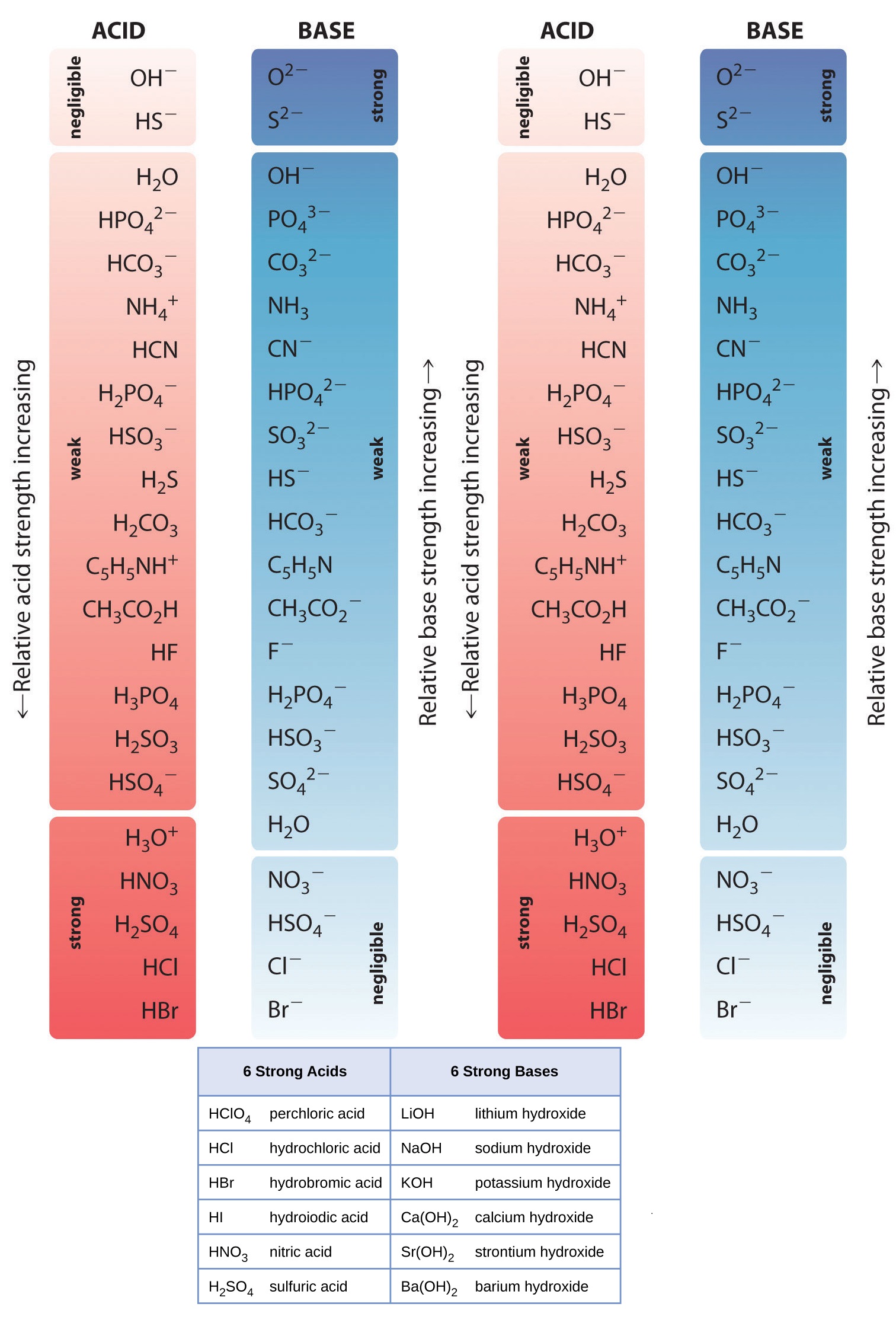
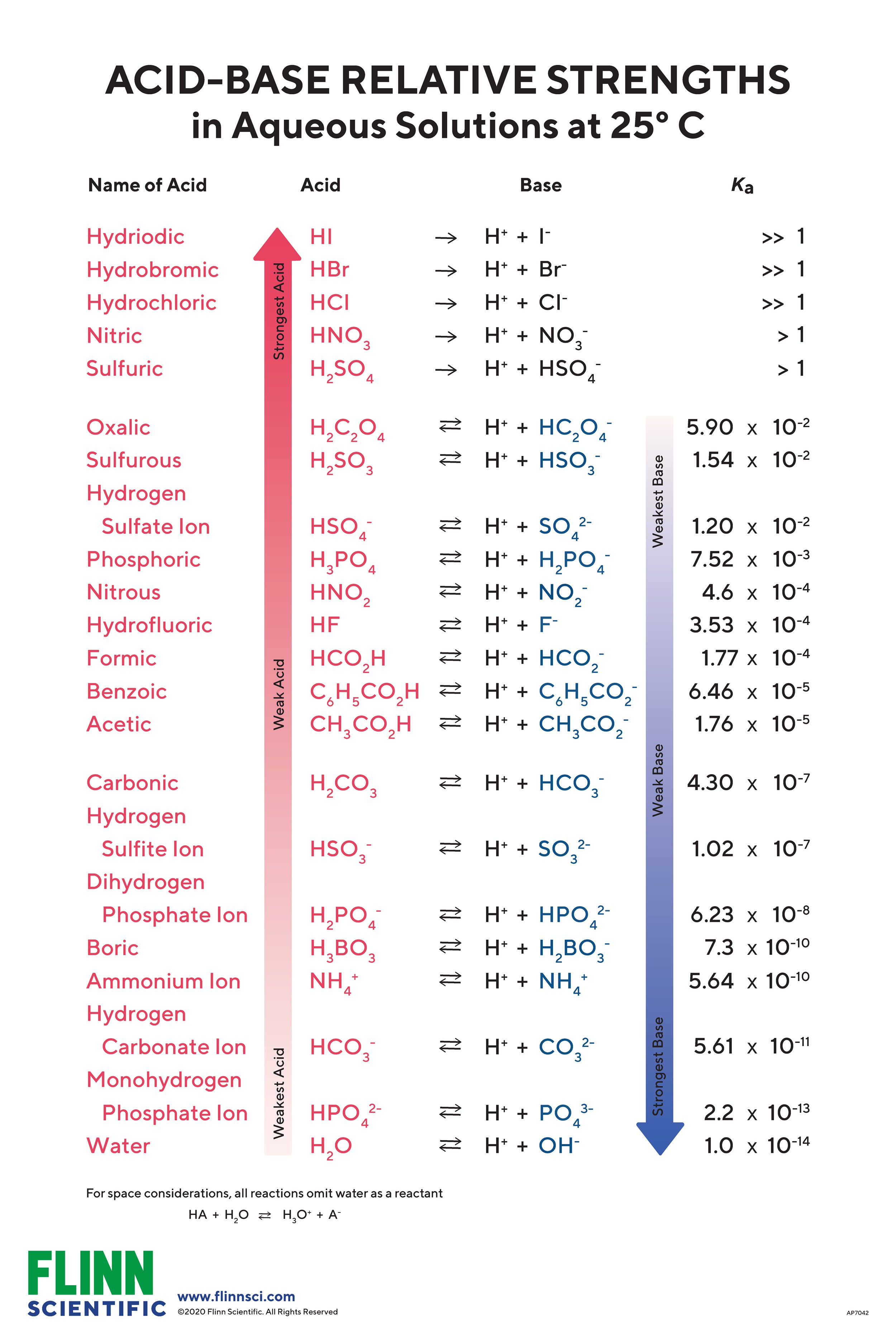
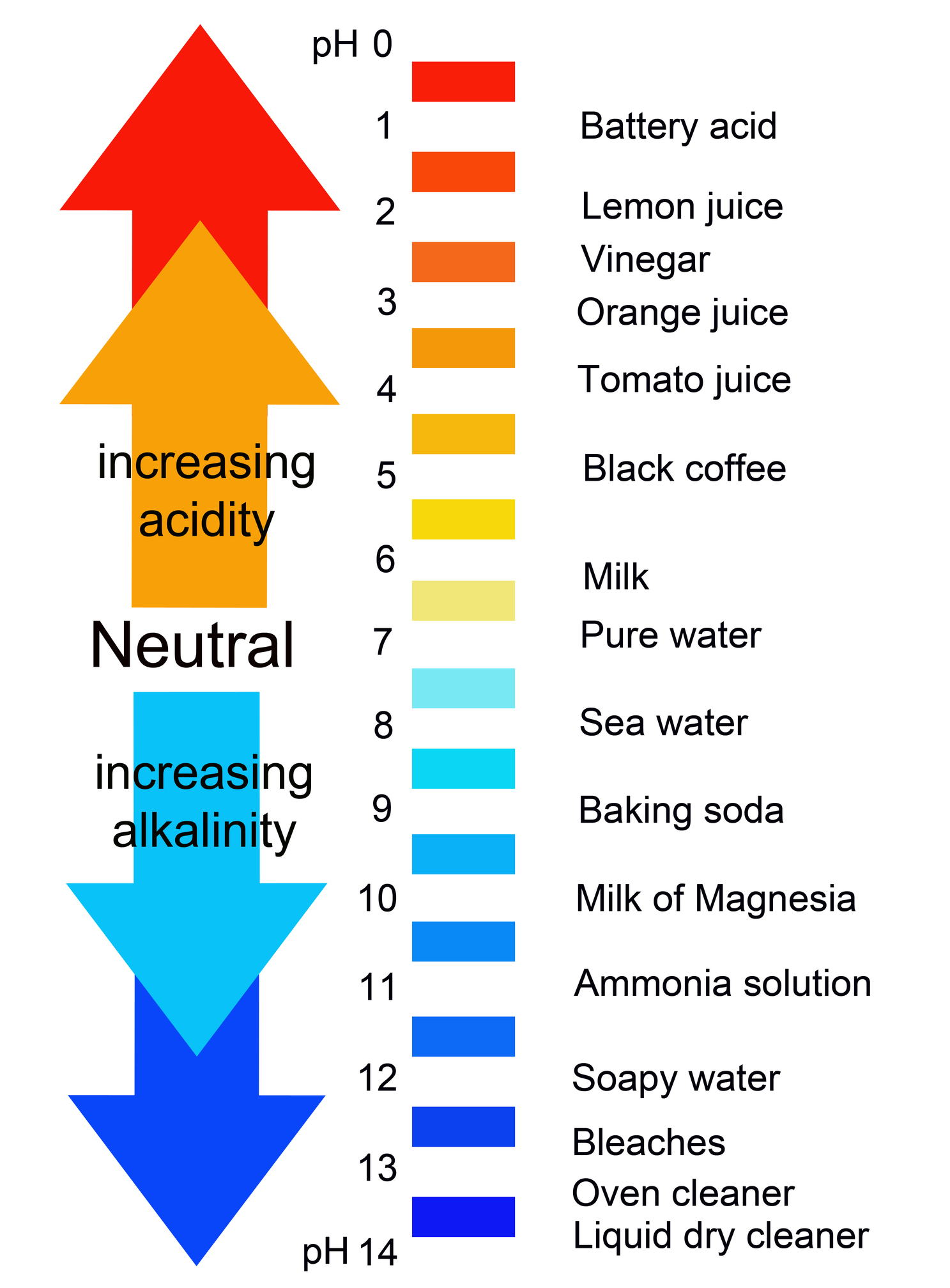
Acid Base Chart - Solutions are classified as acidic or basic based on their hydrogen ion concentration relative to pure water. The strong bases are listed at the bottom right of the table and get weaker as we move to the top of the table. Web use this acids and bases chart to find the relative strength of the most common acids and bases. The strong acids ionize completely in water to yield or or more protons per acid molecule. A ph less than 7 indicates acidity, while a ph greater than 7 indicates alkalinity. Web the seven common strong acids listed from strongest to weakest are: Web rules and resources. Acidic solutions have a higher h + concentration than water (greater than 1 × 10 − 7 m), while basic (alkaline) solutions have a lower h + concentration (less than 1 × 10 − 7 m). Web acid with values less than one are considered weak. Chart or notebook size available. Acidic solutions have a higher h + concentration than water (greater than 1 × 10 − 7 m), while basic (alkaline) solutions have a lower h + concentration (less than 1 × 10 − 7 m). Perchloric (hclo4), hydroiodic (hi), hydrobromic (hbr), hydrochloric (hcl), sulfuric (h2so4), nitric (hno3), and chloric. 48 × 32, each (ap7042) $36.59. Let’s start off with the ph value. The ph scale, ranging from 0 to 14, measures the acidity or alkalinity (basicity) of a solution. There aren’t very many, so it’s a good idea to memorize them, if you can. Web the seven common strong acids listed from strongest to weakest are: (select option to see volume pricing availability) 8½ × 11, pad of 30 (ap7229) $17.50. Web this is a list of the strong acids and strong bases. Web in this tutorial, you will learn about the distinctive properties between acids and bases, what defines an acid vs base, and also what is an amphoteric compound. There aren’t very many, so it’s a good idea to memorize them, if you can. Perchloric (hclo4), hydroiodic (hi), hydrobromic (hbr), hydrochloric (hcl), sulfuric (h2so4), nitric (hno3), and chloric. The strong acids ionize completely in water to yield or or more protons per acid molecule. A ph less than 7 indicates acidity, while a ph greater than 7 indicates alkalinity.. (select option to see volume pricing availability) 8½ × 11, pad of 30 (ap7229) $17.50. There aren’t very many, so it’s a good idea to memorize them, if you can. A ph of 7 is neutral. Web acid with values less than one are considered weak. The strong acids ionize completely in water to yield or or more protons per. Web in this tutorial, you will learn about the distinctive properties between acids and bases, what defines an acid vs base, and also what is an amphoteric compound. Web this is a list of the strong acids and strong bases. There aren’t very many, so it’s a good idea to memorize them, if you can. Acidic solutions have a higher. Web rules and resources. The strong acids ionize completely in water to yield or or more protons per acid molecule. Web in this tutorial, you will learn about the distinctive properties between acids and bases, what defines an acid vs base, and also what is an amphoteric compound. Perchloric (hclo4), hydroiodic (hi), hydrobromic (hbr), hydrochloric (hcl), sulfuric (h2so4), nitric (hno3),. A ph less than 7 indicates acidity, while a ph greater than 7 indicates alkalinity. There aren’t very many, so it’s a good idea to memorize them, if you can. Web in this tutorial, you will learn about the distinctive properties between acids and bases, what defines an acid vs base, and also what is an amphoteric compound. Web rules. Web this is a list of the strong acids and strong bases. Web acid with values less than one are considered weak. 48 × 32, each (ap7042) $36.59. A ph less than 7 indicates acidity, while a ph greater than 7 indicates alkalinity. Web use this acids and bases chart to find the relative strength of the most common acids. 48 × 32, each (ap7042) $36.59. Web in this tutorial, you will learn about the distinctive properties between acids and bases, what defines an acid vs base, and also what is an amphoteric compound. Web rules and resources. The strong bases are listed at the bottom right of the table and get weaker as we move to the top of. Web in this tutorial, you will learn about the distinctive properties between acids and bases, what defines an acid vs base, and also what is an amphoteric compound. (select option to see volume pricing availability) 8½ × 11, pad of 30 (ap7229) $17.50. Acidic solutions have a higher h + concentration than water (greater than 1 × 10 − 7. Web in this tutorial, you will learn about the distinctive properties between acids and bases, what defines an acid vs base, and also what is an amphoteric compound. The strong acids ionize completely in water to yield or or more protons per acid molecule. Let’s start off with the ph value. Web acid with values less than one are considered. Web this is a list of the strong acids and strong bases. Pure water is an example of a substance with a neutral ph. Web acid with values less than one are considered weak. Web the seven common strong acids listed from strongest to weakest are: Solutions are classified as acidic or basic based on their hydrogen ion concentration relative. Solutions are classified as acidic or basic based on their hydrogen ion concentration relative to pure water. The ph scale, ranging from 0 to 14, measures the acidity or alkalinity (basicity) of a solution. Web this is a list of the strong acids and strong bases. Web acid with values less than one are considered weak. There aren’t very many, so it’s a good idea to memorize them, if you can. Pure water is an example of a substance with a neutral ph. A ph less than 7 indicates acidity, while a ph greater than 7 indicates alkalinity. Web use this acids and bases chart to find the relative strength of the most common acids and bases. Web the seven common strong acids listed from strongest to weakest are: A ph of 7 is neutral. (select option to see volume pricing availability) 8½ × 11, pad of 30 (ap7229) $17.50. Let’s start off with the ph value. Web rules and resources. The strong bases are listed at the bottom right of the table and get weaker as we move to the top of the table. The strong acids ionize completely in water to yield or or more protons per acid molecule. 48 × 32, each (ap7042) $36.59.16.5 Strong Acids and Bases Chemistry LibreTexts
What’s The Reaction Between An Acid And A Base?
List of Strong Acids & Bases in Order StudyPK
AcidBase Strength Charts for Chemistry
pH Of Acids And Bases Calculate pH Value Chemistry Byju's
Acids and Bases
Back to Basics Acids, Bases & the pH Scale Precision Laboratories
Water, Acids, and Bases CK12 Foundation
Interpretation Acid Base Tutorial
Acid and Base Chart — Table of Acids & Bases
Web In This Tutorial, You Will Learn About The Distinctive Properties Between Acids And Bases, What Defines An Acid Vs Base, And Also What Is An Amphoteric Compound.
Chart Or Notebook Size Available.
Perchloric (Hclo4), Hydroiodic (Hi), Hydrobromic (Hbr), Hydrochloric (Hcl), Sulfuric (H2So4), Nitric (Hno3), And Chloric.
Acidic Solutions Have A Higher H + Concentration Than Water (Greater Than 1 × 10 − 7 M), While Basic (Alkaline) Solutions Have A Lower H + Concentration (Less Than 1 × 10 − 7 M).
Related Post: