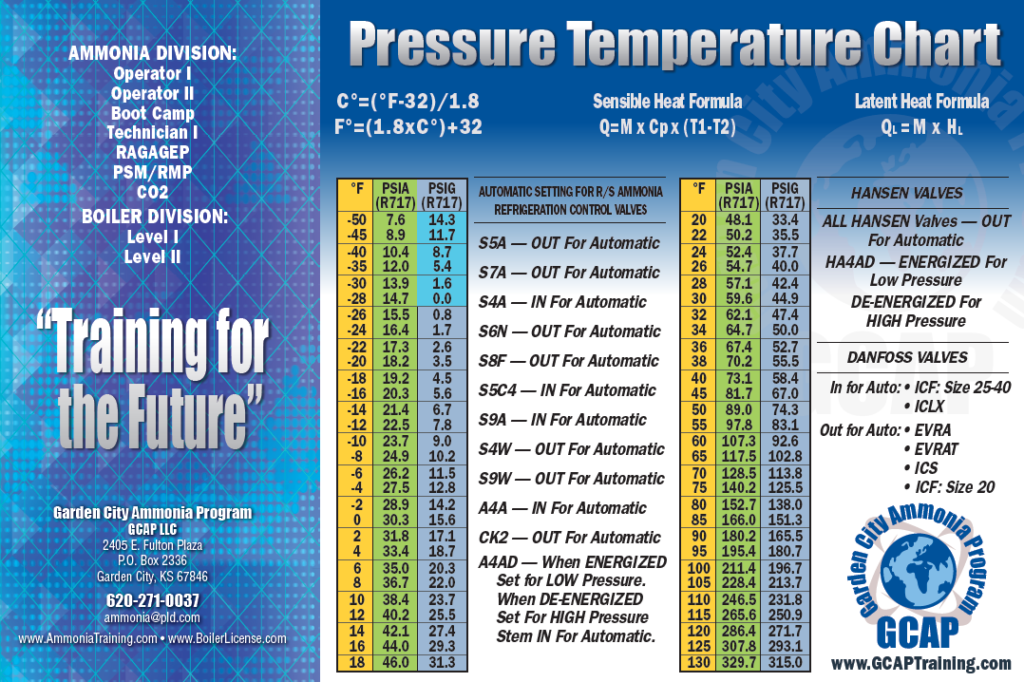
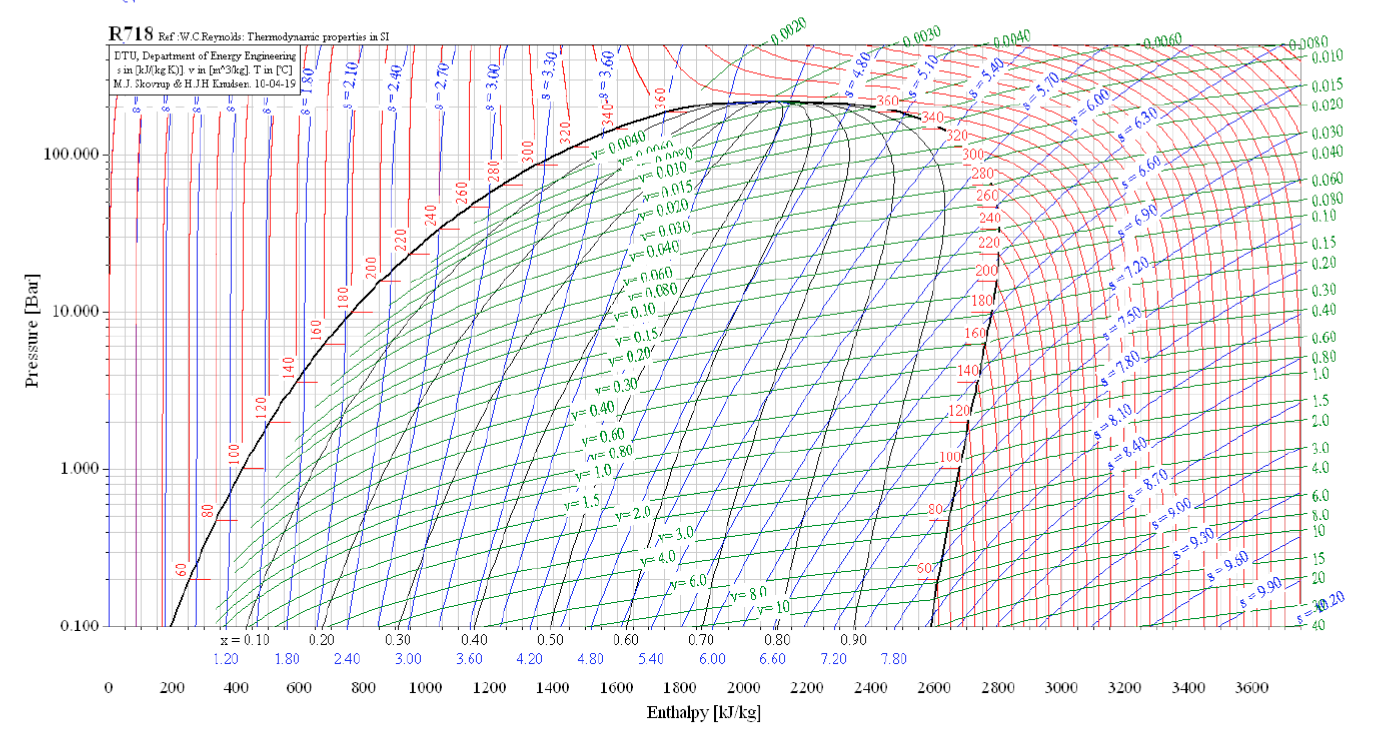
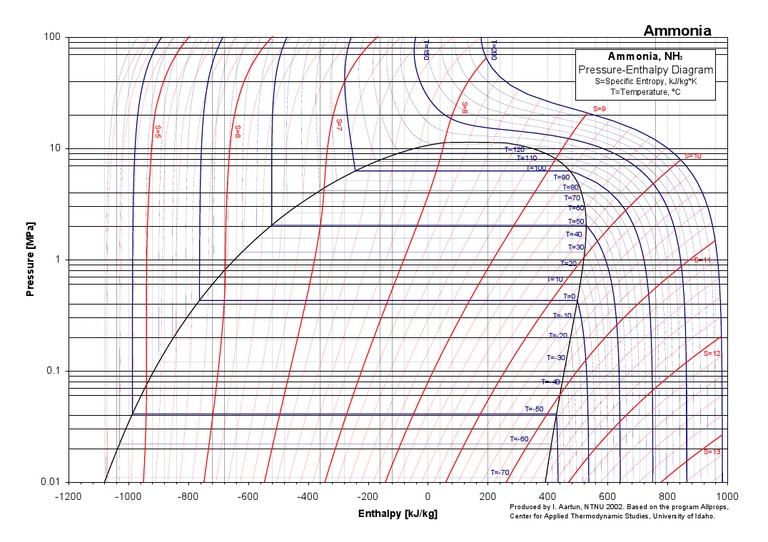
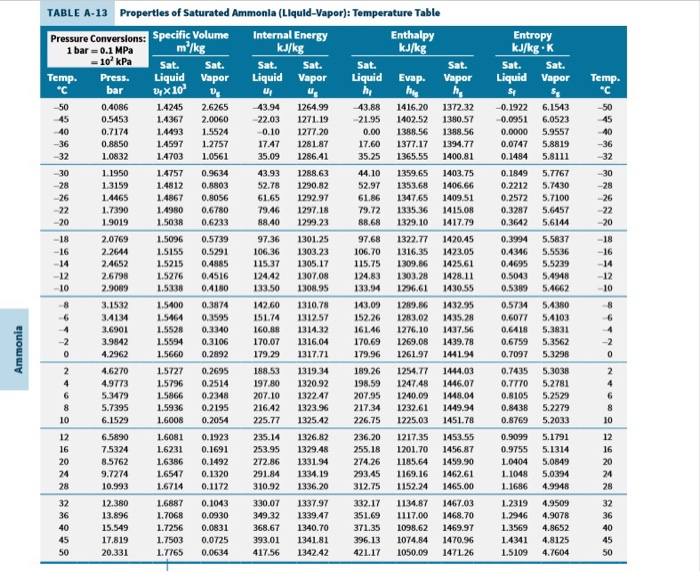
Ammonia Pressure Temperature Chart
Ammonia Pressure Temperature Chart - Web are 0.2% in density, except in the critical region. Both suction and liquid line values are based on a pressure drop equivalent to 1°f change in saturation temperature. For ammonia (nh 3) italicized pressure values (in red) represent inches of mercury (vacuum). Web the chart provides reference values for the pressure of ammonia under different temperature conditions. Web ammonia boiling and condensing pressure / temperature chart. Web refrigerants 22, 134a, 404a, and 507 values are based on 100°f liquid temperature and the stated evaporator temperature. Included are saturation temperatures, corresponding pressures (both gauge and absolute), and enthalpy of both saturated liquid and vapor. R = 0.488198579 kj/ (kg·k) specific gas constant: Ammonia refrigeration and oil separation with reciprocating compressors Web reference states, default for fluid. Web the chart provides reference values for the pressure of ammonia under different temperature conditions. Web reference states, default for fluid. Enthalpy h = 1699.663687 kj/kg at 26.9 c and 0.0010 mpa. Web ammonia boiling and condensing pressure / temperature chart. R = 0.116588 btu/ (lb·°f) 2. For ammonia (nh 3) italicized pressure values (in red) represent inches of mercury (vacuum). R = 0.488198579 kj/ (kg·k) specific gas constant: This document contains a chart listing the pressure and temperature values for ammonia (r717) at various temperature levels between. As an operator/technician you will be using many different saturation charts for the various refrigerants and is an essential tool to understand what is going on inside the system. Refrigerant 717 (ammonia) values are based on 86°f liquid temperature and 20°f evaporator temperature. R = 0.116588 btu/ (lb·°f) 2. Refrigerant 717 (ammonia) values are based on 86°f liquid temperature and 20°f evaporator temperature. Pressure is the base of. Web are 0.2% in density, except in the critical region. Web reference states, default for fluid. The uncertainty in vapor pressure is 0.2%. Web r717 (ammonia) pressure enthalpy chart. (°f) (psia) liquid vapor liquid vapor (lbm/ft3) (ft3/lbm) Friend, “thermophysical properties of fluid systems” in nist chemistry webbook, nist standard reference database number. R = 0.116588 btu/ (lb·°f) 2. Enthalpy h = 1699.663687 kj/kg at 26.9 c and 0.0010 mpa. This document contains a chart listing the pressure and temperature values for ammonia (r717) at various temperature levels between. Web ammonia pressure / temperature chart. As an operator/technician you will be using many different saturation charts for the various refrigerants and is an essential tool to understand what is. Pressure is the base of. R = 0.488198579 kj/ (kg·k) specific gas constant: Web ammonia pressure / temperature chart. Web refrigerants 22, 134a, 404a, and 507 values are based on 100°f liquid temperature and the stated evaporator temperature. Web are 0.2% in density, except in the critical region. Refrigerant 717 (ammonia) values are based on 86°f liquid temperature and 20°f evaporator temperature. Web the chart provides reference values for the pressure of ammonia under different temperature conditions. As an operator/technician you will be using many different saturation charts for the various refrigerants and is an essential tool to understand what is going on inside the system. Web the. Web are 0.2% in density, except in the critical region. Web the following pages provide the properties of ammonia at saturation conditions. Friend, “thermophysical properties of fluid systems” in nist chemistry webbook, nist standard reference database number. (°f) (psia) liquid vapor liquid vapor (lbm/ft3) (ft3/lbm) Enthalpy h = 1699.663687 kj/kg at 26.9 c and 0.0010 mpa. For ammonia (nh 3) italicized pressure values (in red) represent inches of mercury (vacuum). Ammonia saturation or pressure/temperature charts are commonly also known as boiling/condensing charts. (°f) (psia) liquid vapor liquid vapor (lbm/ft3) (ft3/lbm) As an operator/technician you will be using many different saturation charts for the various refrigerants and is an essential tool to understand what is going on. Web refrigerants 22, 134a, 404a, and 507 values are based on 100°f liquid temperature and the stated evaporator temperature. Friend, “thermophysical properties of fluid systems” in nist chemistry webbook, nist standard reference database number. As an operator/technician you will be using many different saturation charts for the various refrigerants and is an essential tool to understand what is going on. For ammonia (nh 3) italicized pressure values (in red) represent inches of mercury (vacuum). Ammonia saturation or pressure/temperature charts are commonly also known as boiling/condensing charts. Web ammonia pressure / temperature chart. In cases where the pressure is below atmospheric, the gauge pressure is italicized and expressed in inches of mercury vacuum. Refrigerant 717 (ammonia) values are based on 86°f. Web ammonia pressure / temperature chart. Both suction and liquid line values are based on a pressure drop equivalent to 1°f change in saturation temperature. This document contains a chart listing the pressure and temperature values for ammonia (r717) at various temperature levels between. Web here’s your free chart. Pressure is the base of. (°f) (psia) liquid vapor liquid vapor (lbm/ft3) (ft3/lbm) Web the following pages provide the properties of ammonia at saturation conditions. R = 0.488198579 kj/ (kg·k) specific gas constant: Ammonia saturation or pressure/temperature charts are commonly also known as boiling/condensing charts. As an operator/technician you will be using many different saturation charts for the various refrigerants and is an essential tool to understand what is going on inside the system. Both suction and liquid line values are based on a pressure drop equivalent to 1°f change in saturation temperature. This document contains a chart listing the pressure and temperature values for ammonia (r717) at various temperature levels between. Web here’s your free chart. The temperature and pressure at which the three phases (gas, liquid, and solid) of a substance coexist in thermodynamic equilibrium triple point pressure of ammonia: Web refrigerants 22, 134a, 404a, and 507 values are based on 100°f liquid temperature and the stated evaporator temperature. Web anhydrous ammonia , nh 3: Ammonia refrigeration and oil separation with reciprocating compressors Web ammonia boiling and condensing pressure / temperature chart. Refrigerant 717 (ammonia) values are based on 86°f liquid temperature and 20°f evaporator temperature. Web ammonia pressure / temperature chart. In cases where the pressure is below atmospheric, the gauge pressure is italicized and expressed in inches of mercury vacuum.Ammonia Pressure / Temperature Chart Online Industrial Training
R717 PressureTemperature Poster Ammonia Refrigeration Training
Ammonia Temperature Pressure Chart
Ammonia Pressure Temperature Chart
Ammonia Pressure Temperature Chart
Ammonia Pressure Chart
Ammonia Temperature Pressure Chart Toolbox
Ammonia Pressure Temperature Chart
Ammonia Temperature Pressure Chart
Ammonia Temperature Pressure Chart
For Ammonia (Nh 3) Italicized Pressure Values (In Red) Represent Inches Of Mercury (Vacuum).
Pressure Is The Base Of.
Friend, “Thermophysical Properties Of Fluid Systems” In Nist Chemistry Webbook, Nist Standard Reference Database Number.
Included Are Saturation Temperatures, Corresponding Pressures (Both Gauge And Absolute), And Enthalpy Of Both Saturated Liquid And Vapor.
Related Post: