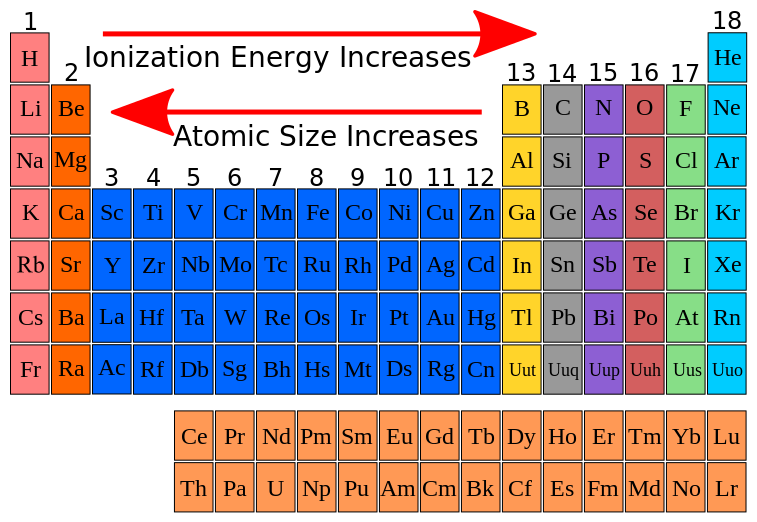
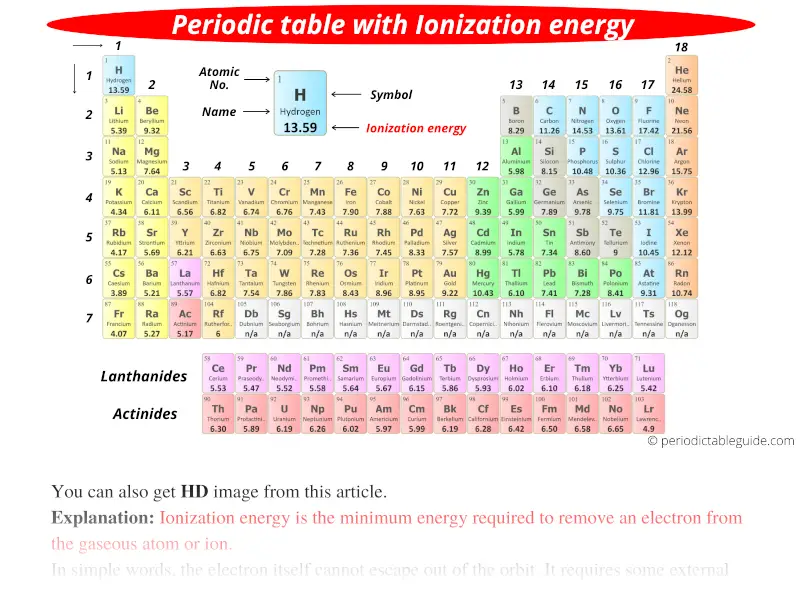
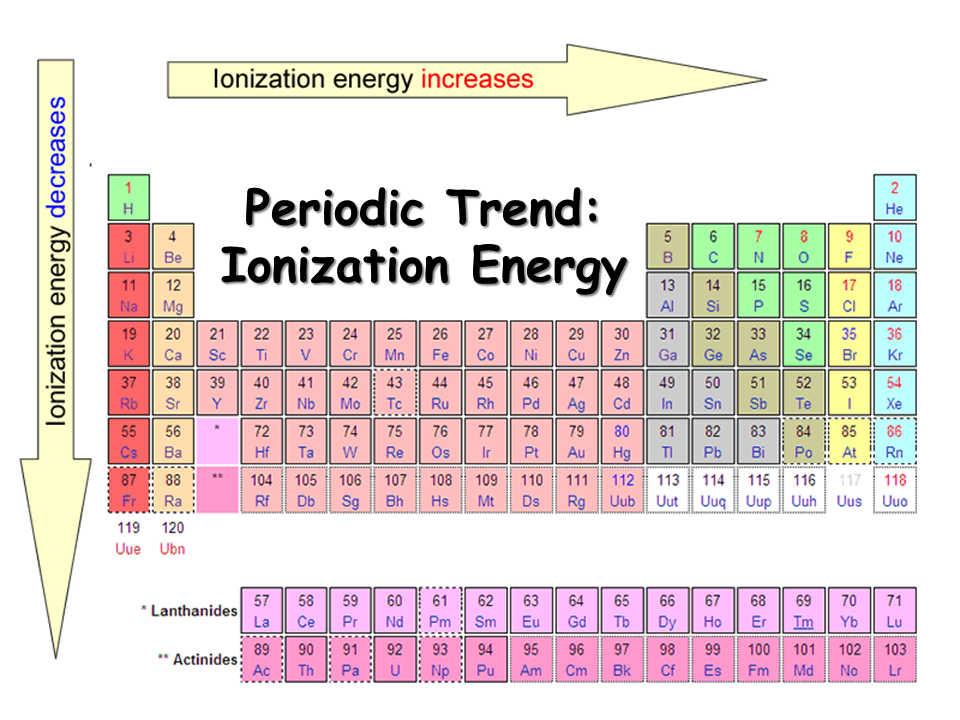
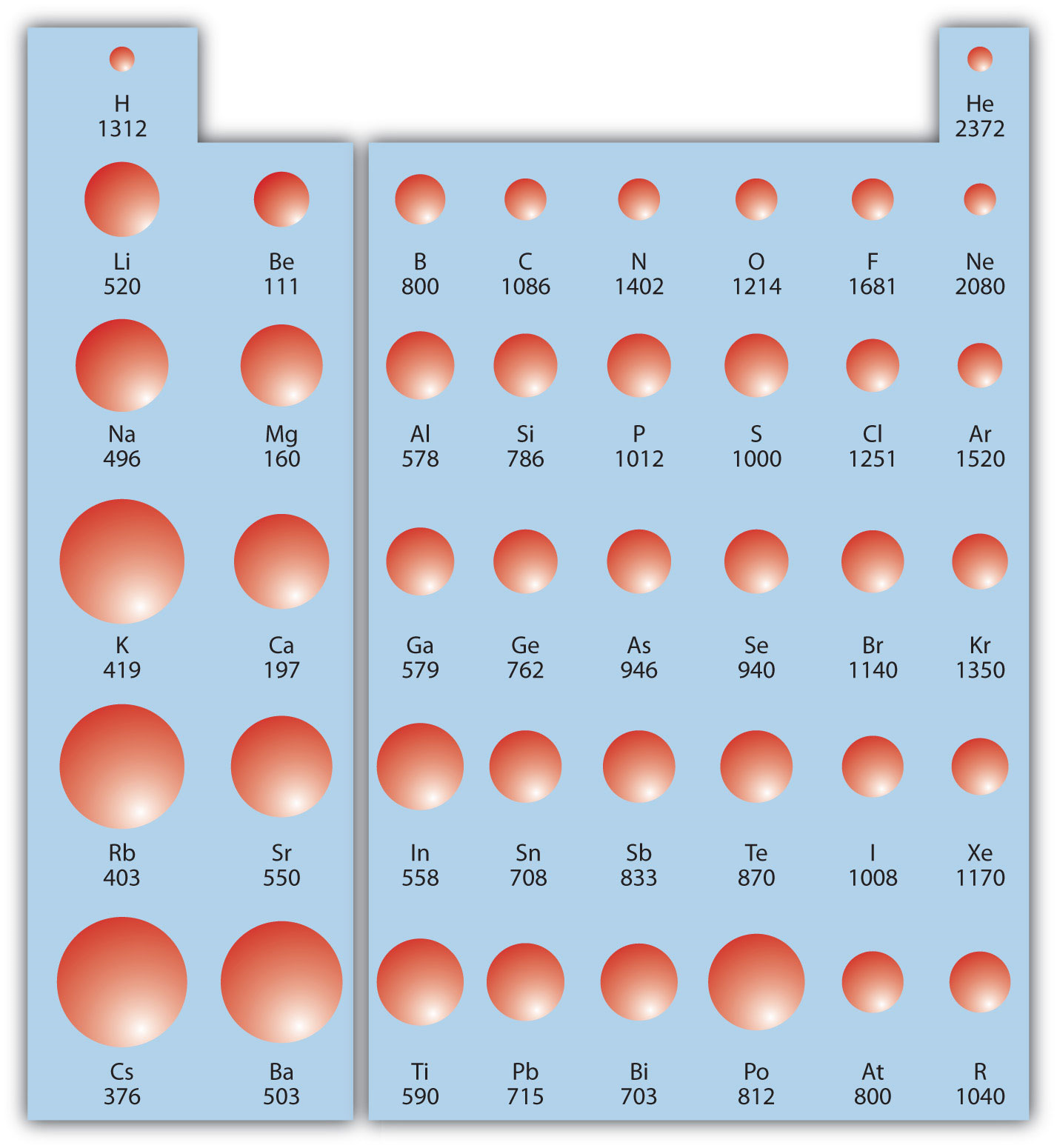
Ionic Energy Chart
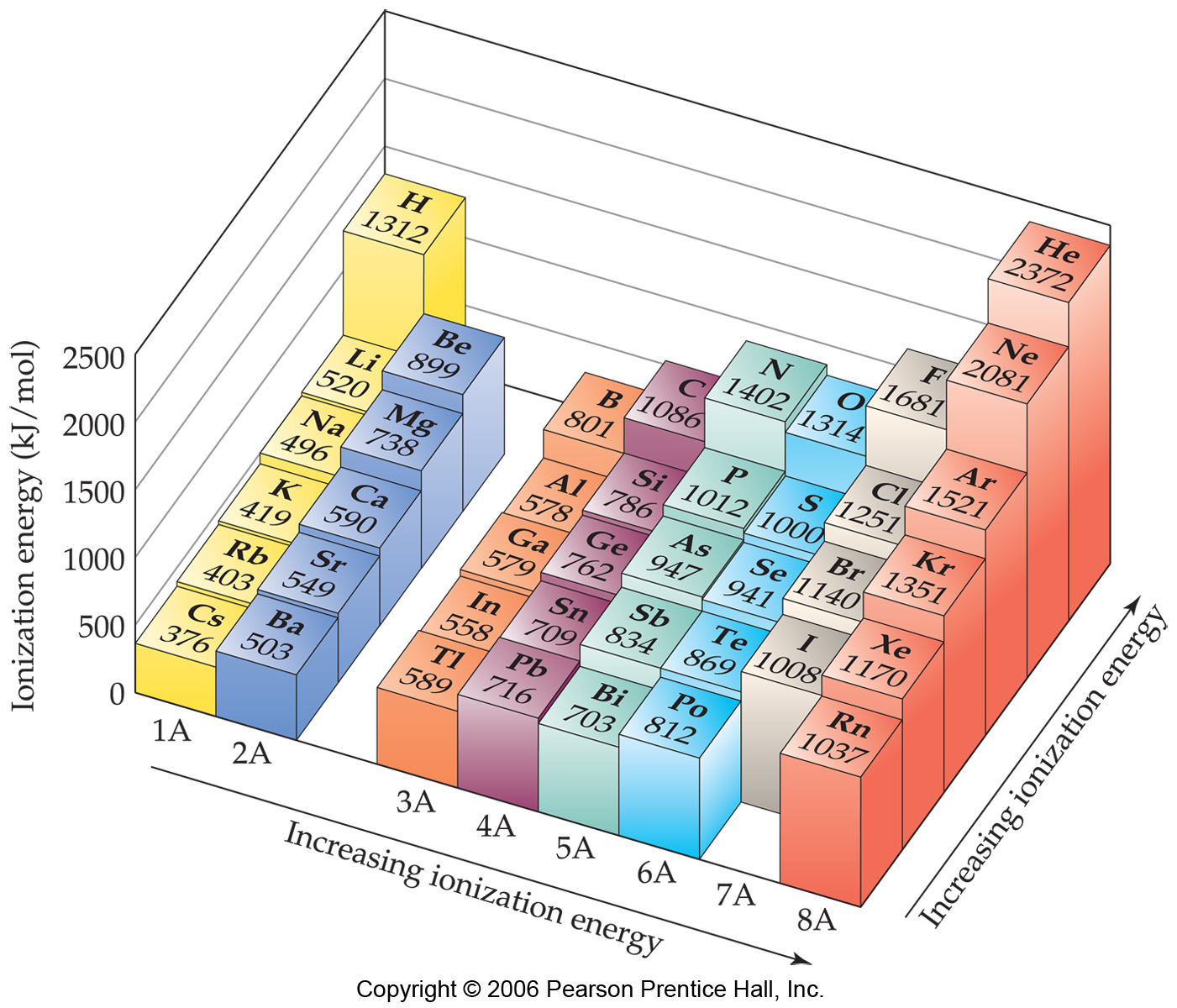
Ionic Energy Chart - Web molar ionization energies of the elements. Web for each atom, the column marked 1 is the first ionization energy to ionize the neutral atom, the column marked 2 is the second ionization energy to remove a second electron. Ionization energy is always positive. The energy required to remove an electron is the ionization energy. Web ionization energy is the minimum energy required to remove an electron from an atom or ion in the gas phase. Web the periodic table of the elements (with ionization energies) element name. The most common units of ionization energy are. A high value of ionization energy. Web ionization energy, in chemistry and physics, the amount of energy required to remove an electron from an isolated atom or molecule. Web ionization energy is the energy required to remove an electron from a neutral atom in its gaseous phase. The energy required to remove the outermost electron from an atom or a positive ion in its ground level. Web ionization energy is a measure of the energy needed to pull a particular electron away from the attraction of the nucleus. Web ionization energy, in chemistry and physics, the amount of energy required to remove an electron from an isolated atom or molecule. One is that when electrons start to fill p orbital the ionization energy goes down a little. Ionization energy is always positive. On the periodic table, first. As you can see on the graph, the noble gases have the highest ionization energies, and the alkali metals have the lowest ionization. Hundreds of chemical reactions in your body involve magnesium. On the periodic table, first. Web ionization is the process of removing an electron from a neutral atom (or compound). Web molar ionization energies of the elements. A high value of ionization energy. Web when electrons are removed in succession from an element, the transition from removing valence electrons to removing core electrons results in a large jump in ionization. Web ionization energy is a measure of the energy needed to pull a particular electron away from the attraction of. Web looking at the graph of ionization energies, it is clear that indium(atomic number 49) does have a lower ionization energy than cadmium (atomic number 48),. Web the periodic table trend on a graph. One is that when electrons start to fill p orbital the ionization energy goes down a little. Web ionization energy is the minimum energy required to. The energy required to remove an electron is the ionization energy. Web molar ionization energies of the elements. The stronger an electron is bound to an atom the more. Web looking at the graph of ionization energies, it is clear that indium(atomic number 49) does have a lower ionization energy than cadmium (atomic number 48),. Web ionization is the process. Web an element's first ionization energy is the energy required to remove the outermost, or least bound, electron from a neutral atom of the element. Web the ionization energy of atoms, denoted e i, is measured by finding the minimal energy of light quanta or electrons accelerated to a known energy that will kick out the least bound. The most. Web explore how ionization energy changes with atomic number in the periodic table of elements via interactive plots. Web when electrons are removed in succession from an element, the transition from removing valence electrons to removing core electrons results in a large jump in ionization. Web magnesium is one of the most abundant minerals in your body. The energy required. As you can see on the graph, the noble gases have the highest ionization energies, and the alkali metals have the lowest ionization. On the periodic table, first. Web chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the gaseous atom \(e\) in its ground state. Web explore how. Your body needs it for muscle. The energy required to remove the outermost electron from an atom or a positive ion in its ground level. Web explore how ionization energy changes with atomic number in the periodic table of elements via interactive plots. Web chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to. Web ionization energy chart of all the elements is given below. As you can see on the graph, the noble gases have the highest ionization energies, and the alkali metals have the lowest ionization. Web an element's first ionization energy is the energy required to remove the outermost, or least bound, electron from a neutral atom of the element. The. Web ionization energy chart of all the elements is given below. Web there are couple of reasons for that. Web for each atom, the column marked 1 is the first ionization energy to ionize the neutral atom, the column marked 2 is the second ionization energy to remove a second electron. Web ionization energy is a measure of the energy. The first ionization energy, second. Web the ionization energy of atoms, denoted e i, is measured by finding the minimal energy of light quanta or electrons accelerated to a known energy that will kick out the least bound. Web ionization energy is the quantity of energy that an isolated, gaseous atom in the ground electronic state must absorb to discharge. The table lists only the first ie in ev units. Hundreds of chemical reactions in your body involve magnesium. Web ionization energy is the amount of energy needed to remove an electron from a neutral gaseous atom and form an ion. Web there are couple of reasons for that. Web chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the gaseous atom \(e\) in its ground state. As you can see on the graph, the noble gases have the highest ionization energies, and the alkali metals have the lowest ionization. Web ionization energy chart of all the elements is given below. Web looking at the graph of ionization energies, it is clear that indium(atomic number 49) does have a lower ionization energy than cadmium (atomic number 48),. These tables list values of molar ionization energies, measured in kj⋅mol −1. The energy required to remove an electron is the ionization energy. Another is when each of 3 p orbitals have one. Web explore how ionization energy changes with atomic number in the periodic table of elements via interactive plots. The ionization energy associated with. Web ionization energy is a measure of the energy needed to pull a particular electron away from the attraction of the nucleus. Web ionization energy is the quantity of energy that an isolated, gaseous atom in the ground electronic state must absorb to discharge an electron, resulting in a cation. Web the periodic table of the elements (with ionization energies) element name.FileIonization energy atomic size.svg Wikimedia Commons
Periodic table with Ionization Energy Values (Labeled Image)
Ionization Enthalpy NEET Lab
9.9 Periodic Trends Atomic Size, Ionization Energy, and Metallic
Periodic Trends in Ionization Energy Chemistry Socratic
Example
Ionization Energy Definition, Chart & Periodic Table Trend
What Is Ionization Energy? Definition and Trend
Periodic Table Ionization Energy Labeled
Periodic Table Ionization Energy Chart
First Ionization Energy, Second Ionization Energy As Well As Third Ionization Energy Of The Elements Are.
Web Ionization Is The Process Of Removing An Electron From A Neutral Atom (Or Compound).
Ionization Energy Is Always Positive.
Your Body Needs It For Muscle.
Related Post: